About Us
It is a long established fact that a reader will be distracted by the readable content of a page when looking at its layout.

It is a long established fact that a reader will be distracted by the readable content of a page when looking at its layout.


AnaBios offers high-quality human tissue and cells recovered through our extensive network of hospitals and organ procurement organizations. We utilize proprietary methods, practices and streamlined logistics to maximize the preservation of physiological function. Our goal is to deliver industry-leading human tissues and cells for scientific research and drug discovery.
AnaBios creates the unprecedented opportunity for researchers to develop safer, more effective drugs while reducing time-to-market and clinical development costs. Accordingly, we have developed proprietary processes for functional ex vivo human platforms that redefine the concept of “First in Human” studies and bring true human biology to early pre-clinical discovery.

AnaBios announced the acquisition of Cell Systems, a human primary cell and cell culture media company located in Kirkland, Washington. The purchase of Cell Systems will further enhance AnaBios’ unique human tissue and cells portfolio and help scientists expedite drug discovery and further their understanding of cell biology through access to an expansive array of biologically relevant tools.
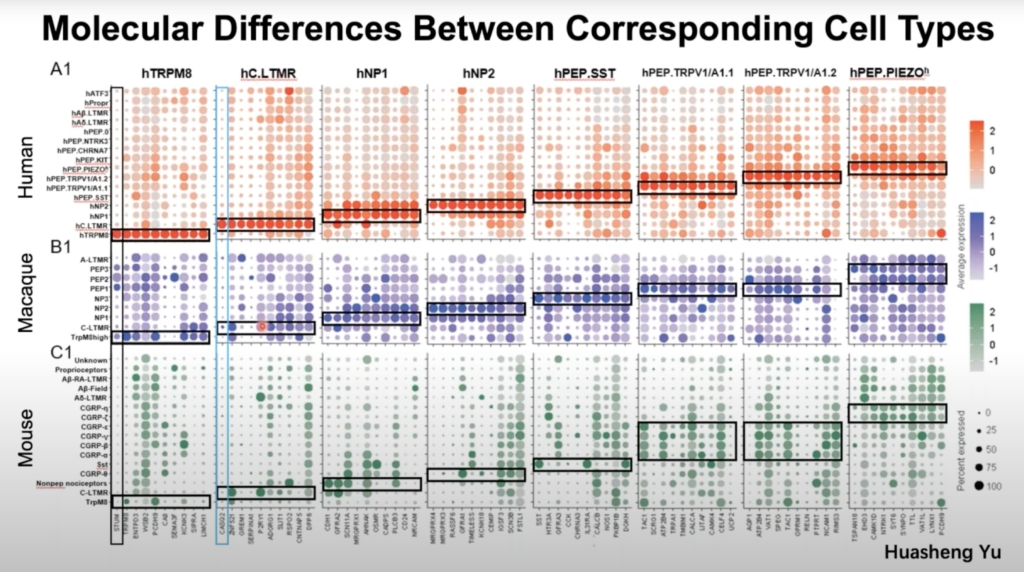
Primary somatosensory neurons in the DRG play critical roles in sensing external and internal stimuli and generate sensations like pain, itch, warm, cold and touch. Most knowledge regarding DRG neurons comes from model organisms, which may have significant species differences from humans. Dr. Wenqin Luo, Associate Professor of Neuroscience at the University of Pennsylvania, discuss her research team’s work developing a new approach by isolating individual somas of human DRG neurons using laser capture microdissection and performing high-depth Smart-seq2 RNAseq.
To request a quote for human tissue and/or services, please complete the form below. We will respond to you in 24-48 business hours.
Our sales team will respond to your inquiry in 24 hours. In the meantime, please note that by clicking on the "Submit" button, you are agreeing to AnaBios' Privacy Policy.